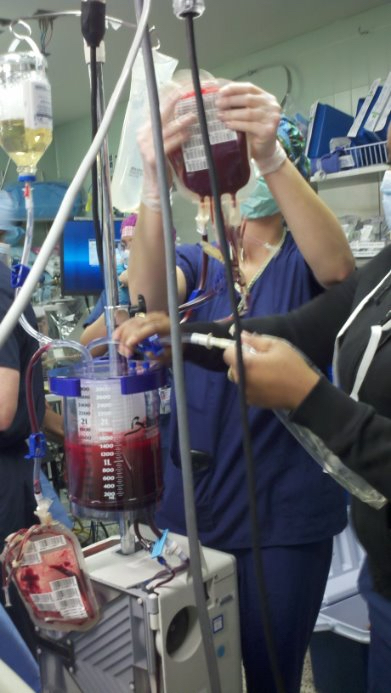
MATIC-2 is seeking to improve outcomes due to life-threatening bleeding in children who suffer traumatic injury.
The No. 1 cause of death in children is trauma. There are an estimated 2,000 pediatric deaths from traumatic bleeding in the U.S. each year that are preventable with optimal care, yet there have been no large-scale clinical trials to guide the best way to treat children with life-threatening bleeding after traumatic injury.
Massive Transfusion in Children-2 (MATIC-2) is a study aiming to improve trauma care in children.
To do so, children with serious injuries who need emergent care will get one or more treatments that include blood products (component therapy (CT) and low titer group O whole blood (LTOWB)), and medications (Tranexamic Acid (TXA) or salt water). These treatments are already being given to children across the United States, but doctors aren’t sure which bundle of treatments is best. Many studies look at adults, but we don’t always know how to best treat kids.
This study will be completed at about 20 different hospitals in the United States.
These hospitals will be split into four groups based on the four possible treatment combinations. Each group will rotate through all different combinations of care. This design helps to control for differences between sites. Up to 1,000 children will be in the study.
For more information about the MATIC-2 Trial: classic.clinicaltrials.gov/ct2/show/NCT06070350
Methods
Inclusion Criteria
MATIC-2 will only enroll patients who have met the following criteria:
- Children, defined as an estimated age at the time of admission as ≤18 years of age
- Activation of a clinical site’s “Massive Transfusion Protocol”*
- Confirmed or suspected life-threatening traumatic bleeding with at least 2 of the following:
- Hypotension for age (< 5 %tile)
- Tachycardia for age (>95 %tile)
- Traumatic injury with findings consistent with severe bleeding (e.g. penetrating injury, hemothorax, distended abdomen with bruising, amputation of limb.)
Primary Outcome
MATIC-2 will utilize 24-hour mortality as its primary outcome.
Design, Interventions and Sample Size
MATIC-2 has a platform design, which allows for the comparison of multiple combinations of therapies in the same trial. The intervention arms for the MATIC-2 trial are blood products (Low-Titer Group O Whole Blood (LTOWB) versus component therapy (red blood cells, plasma and platelets)) and hemostatic agents (Tranexamic Acid (TXA) versus salt water). We anticipate that 1,000 children will be enrolled across all participating sites.
Consent
Children who are included in MATIC-2 are very sick and require urgent care that cannot be delayed in order to obtain permission from their parent/guardian ahead of time to be a part of the research study. Instead, studies like MATIC-2 use an FDA regulated process called “Exception from Informed Consent” (EFIC).
Read more about EFIC within MATIC-2 →